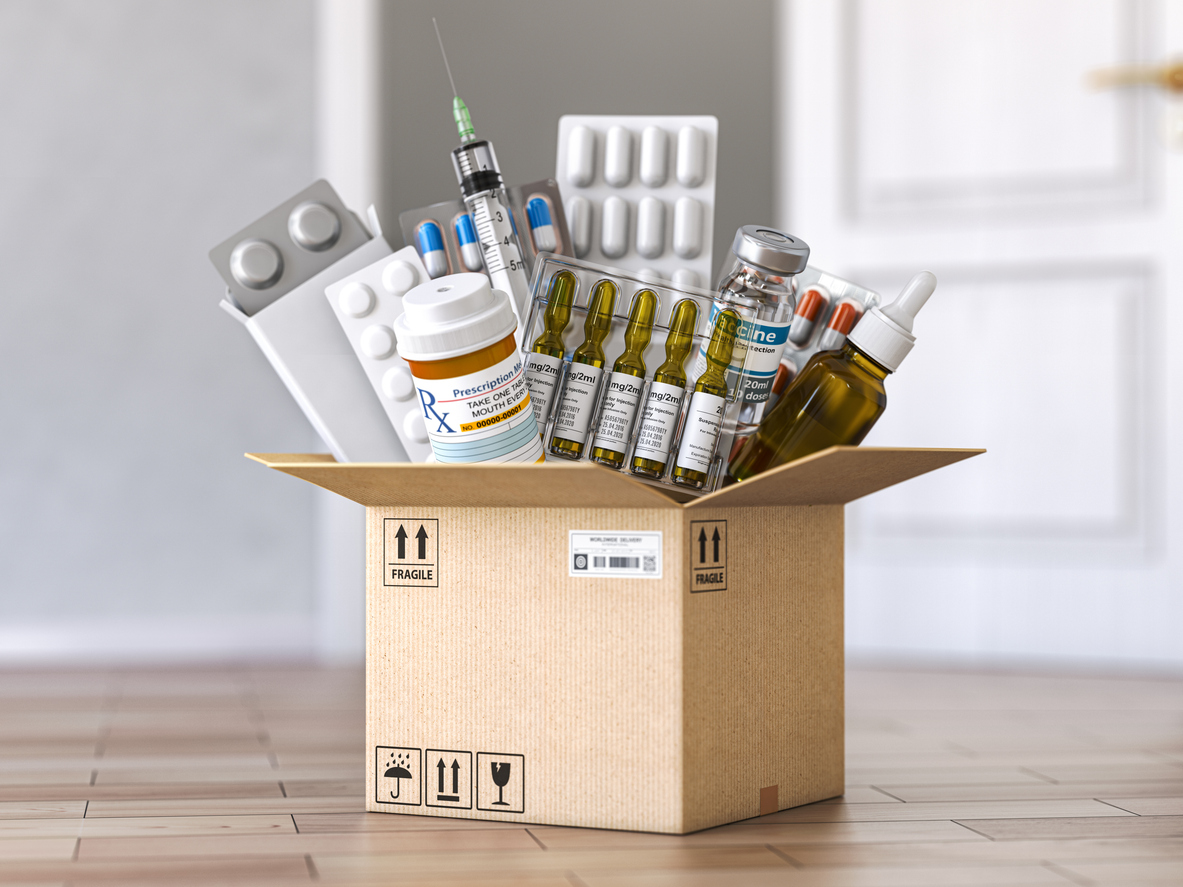
Pharmaceutical Packaging Import from China to UK: A Practical Guide for UK Buyers
The pharmaceutical packaging import from China to UK has grown steadily as UK pharmaceutical companies, contract manufacturers, and healthcare distributors seek cost-effective, compliant, and scalable packaging solutions. China is a major global producer of pharmaceutical glass, plastic containers, blister packs, caps, closures, and secondary packaging materials.
However, importing pharmaceutical packaging into the UK requires more than simply securing competitive pricing. Regulatory compliance, quality assurance, documentation, and reliable logistics are all critical to protecting product integrity and patient safety.
This guide explains what UK importers need to consider when sourcing pharmaceutical packaging from China and how experienced trade partners such as Wigmore Trading can support the process.
Why Source Pharmaceutical Packaging from China?
China has developed a strong manufacturing base for pharmaceutical packaging materials, including:
-
Glass vials and ampoules
-
HDPE and PET bottles
-
Blister foil and PVC/PVDC films
-
Aluminium caps and closures
-
Child-resistant packaging systems
-
Printed cartons and leaflets
Manufacturers in China often offer competitive production costs, large-scale capacity, and advanced production lines that meet international pharmaceutical standards. For UK buyers, this can translate into improved margins and greater supply flexibility.
However, lower costs should never come at the expense of compliance. Pharmaceutical packaging is tightly regulated in the UK, and any imported materials must meet strict safety and quality standards.
Regulatory Requirements for Pharmaceutical Packaging Import from China to UK
When managing a pharmaceutical packaging import from China to UK, compliance with UK regulations is essential. The Medicines and Healthcare products Regulatory Agency (MHRA) oversees medicinal product standards, and packaging materials must meet applicable requirements under UK pharmaceutical legislation.
Key considerations include:
-
Compliance with UK Good Manufacturing Practice (GMP) standards
-
Material conformity with pharmacopoeia requirements (e.g., BP, EP)
-
Migration and extractables testing for plastic materials
-
Tamper-evidence and child-resistance where required
-
Accurate labelling and traceability documentation
Importers must ensure that suppliers provide proper certification, including Certificates of Analysis (CoA), GMP documentation, and technical data sheets. Inadequate documentation can result in customs delays or product rejection.
Wigmore Trading supports UK businesses by coordinating supplier verification, reviewing documentation, and ensuring that packaging imports align with regulatory expectations before shipment.
Quality Control and Supplier Due Diligence
Quality assurance is central to any pharmaceutical packaging import from China to UK. Packaging failures can compromise sterility, stability, and product safety.
Before entering into supply agreements, UK buyers should:
-
Conduct factory audits or request third-party inspection reports
-
Review quality management certifications such as ISO 15378 (GMP for primary packaging materials)
-
Confirm raw material traceability
-
Request product samples for laboratory testing
Pre-shipment inspections are also important. These help confirm that goods meet agreed specifications prior to export, reducing the risk of non-compliant products entering the UK supply chain.
Through its sourcing and supply chain network, Wigmore Trading can help coordinate inspections, manage supplier communications, and reduce the risks associated with overseas procurement.
Shipping and Logistics Considerations
Pharmaceutical packaging materials often require careful handling during transportation to maintain cleanliness and structural integrity.
For a pharmaceutical packaging import from China to UK, buyers must consider:
-
Container loading standards to prevent contamination or damage
-
Moisture protection for cartons and paper-based packaging
-
Appropriate Incoterms and insurance coverage
-
Accurate customs classification (HS codes)
-
UK import duties and VAT obligations
Sea freight is typically the most cost-effective option for bulk shipments, while air freight may be used for urgent orders or smaller consignments. Delays at ports or incomplete paperwork can disrupt production schedules, particularly for manufacturers operating on just-in-time systems.
An experienced logistics partner like Wigmore Trading can coordinate freight forwarding, customs clearance, and documentation management to ensure smooth delivery from Chinese suppliers to UK facilities.
Risk Management in Pharmaceutical Packaging Imports
Supply chain resilience has become increasingly important in recent years. UK pharmaceutical companies importing packaging from China must account for potential risks such as:
-
Production delays
-
Shipping disruptions
-
Regulatory changes
-
Currency fluctuations
Diversifying suppliers or maintaining buffer stock can reduce exposure to unexpected disruptions. Clear contractual agreements covering quality standards, lead times, and liability are also essential.
Wigmore Trading assists clients in structuring reliable sourcing arrangements, negotiating supply terms, and building more resilient import strategies.
Sustainability and Environmental Considerations
Sustainability is becoming a priority across the pharmaceutical sector. When importing packaging from China to the UK, businesses should assess:
-
Recyclability of materials
-
Use of recycled content
-
Compliance with UK packaging waste regulations
-
Extended Producer Responsibility (EPR) requirements
UK importers are responsible for ensuring compliance with packaging waste obligations, including proper reporting and recycling contributions. Working with suppliers who understand sustainable material options can support long-term compliance and corporate responsibility goals.
Conclusion
A successful pharmaceutical packaging import from China to UK requires careful planning, strict quality control, and full regulatory compliance. While China offers competitive manufacturing capacity and product diversity, UK importers must manage documentation, logistics, and risk effectively to protect both their supply chains and end users.
By combining thorough supplier due diligence with structured logistics management and compliance oversight, businesses can benefit from cost efficiencies without compromising safety or standards.
Wigmore Trading can help. Contact Wigmore Trading today to streamline your sourcing.





Comments are closed.